Quality Management
| Key performance indicators (KPIs) |
FY22 Targets |
FY22 Results |
Appraisal |
| Number of accidents (major safety-related accidents) |
23 or fewer |
Internal: 4
Group companies: 3 |
Achieved |
| Number of accidents (major logistics-related accidents) |
70 parts per million (ppm) or fewer |
88ppm |
Not achieved |
| Incidence rate of small-lot transport complaints |
(consolidate to the goal of logistics claim/problem incidence rate) |
| Quality audits conducted for manufacturing contractors |
50% or more of companies targeted |
55% |
Achieved |
| Quality audits conducted for Tosoh domestic consolidated subsidiaries |
Manufacturing companies targeted (24) |
24 |
Achieved |
Basic Concept
Under the RC promotion system, the Tosoh Group strives to enhance customer satisfaction by ensuring comprehensive quality control and logistics safety, reliably operating the quality management system, and providing products and services that reflect customer needs in a timely and stable manner. Moreover, we are committed to continuous quality improvement while complying with legal and regulatory requirements related to our products.
List of RC Activity Target Results: Quality Assurance »
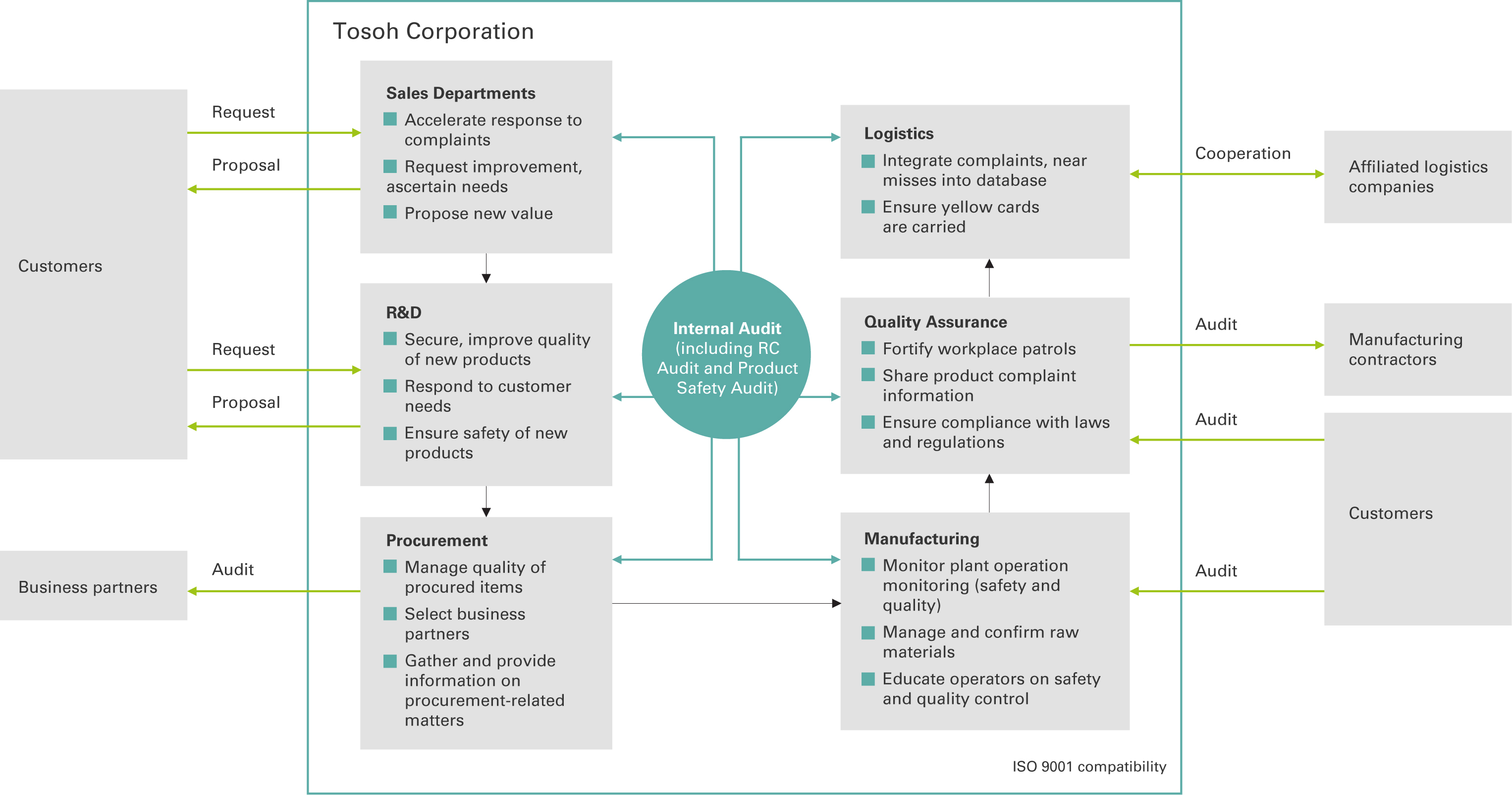
Maintaining and Improving Quality Management
Tosoh Corporation has established a quality management system (QMS) appropriate for its products and organizational structure, and has acquired ISO 9001, ISO 13485, and other certifications to improve product quality and customer satisfaction. Certified complexes and Group companies are subject to internal audits of their activities related to the QMS. This is followed by external audits and management reviews by senior general managers of the complexes and plants to improve the QMS.
Internal Audits
Tosoh conducts internal audits annually within its complexes to make sure the QMS is functioning properly with the aim of constantly improving its operations. Tosoh audited 63 of its departments in fiscal 2022, with themes such as confirmation of the process for receiving raw materials, filling and shipping processes, and the appropriateness of efforts to meet quality targets set by each division. In addition, headquarters conducts RC audits annually at complexes. In fiscal 2022, RC audits were conducted under the themes of reducing product complaints and strengthening supply chain management.
Group Company Support
Parent company Tosoh Corporation supports the quality management of its Group companies and provides opportunities for regular exchange of opinions. Tosoh strives to maintain and improve the quality level of the entire Group by providing advice to suppliers on conducting audits and supporting the strengthening of quality assurance systems. In fiscal 2022, we conducted a voluntary survey of 24 companies regarding their quality control systems. No violations of laws or regulations or any cases that could impact the environment or product safety when used by end users were found in the Tosoh Group.
Supply Chain Initiatives
The Tosoh Group also conducts audits of its major suppliers and manufacturing contractors to confirm the efficacy of their quality management systems, quality control status, status of response to prevent recurrence of complaints, of maintenance and management of measures to prevent recurrence of past nonconformance cases, and of quality compliance activities. In fiscal 2022, Tosoh conducted online and paper document audits of a total of 88 suppliers and production contractors. We are currently working to address issues and problems detected. Moreover, we are striving to strengthen quality control by checking the quality control status of tank locations outside the company.
Supply Chain Management Structure
Product Quality Improvement
Initiatives at Complexes
Each complex has its own policy for maintaining and improving quality, reducing product-related complaints, and increasing customer satisfaction. Complaints that occur at our complexes and group companies are distributed as monthly bulletins to share information and avoid similar complaints and problems.
Moreover, in order to reduce the number of complaints about foreign matter contamination, soiling of product packaging materials, and damage, Tosoh conducts quality site inspections to confirm the status of countermeasures against contamination originating from the manufacturing process, the management status of raw materials, packaging material storage areas, product storage areas, and warehouse facilities. The company further ensures that measures are properly implemented to prevent the recurrence of complaints.
In fiscal 2022, Tosoh followed up on measures to prevent recurrence of nonconformities that occurred in the past, and confirmed the implementation status of measures against foreign matter contamination based on risk assessment, including procedures. The company also conducted QMS training to raise awareness of quality control. There were 11 product complaints. Tosoh will continue our quality workplace inspections to eliminate product complaints and improve quality. There were no product liability incidents or violations of quality-related laws and regulations in fiscal 2022.

Quality patrol
Number of Product Complaints, Product Liability Accidents, and Legal Violations (Tosoh non-consolidated)
|
FY19 |
FY20 |
F21 |
FY22 |
| Product complaints |
50 |
18 |
10 |
11 |
| Product liability accidents |
0 |
0 |
0 |
0 |
| Violations of quality-related laws and regulations |
0 |
0 |
0 |
0 |
Pharmaceutical and Medical Device Initiatives
Tosoh manufactures and sells in vitro diagnostics and medical devices for the diagnosis of various diseases such as heart disease and cancer, lifestyle-related diseases such as diabetes, and infertility treatment. This includes reagents for genetic testing and antigen quantification testing for COVID-19. To manufacture and sell in vitro diagnostic drugs and medical devices, we have obtained manufacturing and sales licenses for in vitro diagnostics and medical devices as stipulated by Japan's Pharmaceuticals and Medical Device Act. We have further established a management system to ensure product efficacy and safety.* In fiscal 2022, we conducted audits at five sites of manufacturers aimed at strengthening the quality assurance system.
*Japan Act on Securing Quality, Efficacy and Safety of Products Including Pharmaceuticals and Medical Devices
List of RC Activity Targets and Achievements: Pharmaceutical Affairs Act »
Reducing Logistics Complaints and Problems
Tosoh's products are delivered to customers mainly by truck and ship. Since some products contain substances such as hazardous and toxic materials that are regulated by law, it is important to manage the cargo from loading, during transportation, and unloading at the delivery destination. Therefore, the Logistics department is taking the initiative in activities aimed at reducing the number of accidents and the annual incidence rate of logistics complaints and problems.*
*Incidence rate = number of logistics accidents, complaints, and problems / number of products transported x 1 million.
Initiatives Toward Reducing Complaints and Problems
The Logistics RC Promotion Committee meets once per quarter to discuss the causes of complaints and problems, as well as countermeasures, and rolls them out groupwide. Tosoh regularly educates, instructs, and audits its logistics subcontractors (Tosoh audited 49 companies in fiscal 2022). In addition to comprehensive manuals and checklists for loading and unloading hazardous and poisonous materials, the company also provides safety education on the chemical product handling. It further surveys hazardous areas at delivery sites and request improvements in the interest of preventing industrial accidents and problems at delivery sites. Tosoh is also working on the hardware side, and actively introducing safety devices into our transportation equipment.
Chemical Substance Management
| Key performance indicators (KPIs) |
FY22 Targets |
FY22 Results |
Appraisal |
| Number of major instances of noncompliance |
0 |
0 |
Achieved |
Basic Concept
Tosoh Corporation promotes activities related to chemical substance management under the RC promotional system. To ensure that customers can use our products safely and with peace of mind, the Tosoh Group ascertains accurate information on chemical substances and provides information on the chemical content of its products. Tosoh collects information and shares the latest updates with its complexes, related departments, and Group companies through briefings, and educational programs to ensure compliance with Japanese and foreign laws and regulations related to chemical substances.
Compliance with Laws and Regulations
When a new product is released on the market, it has to be reported, registered, and quantified according to each country’s laws and regulations. Tosoh is responding to revisions to laws and regulations being implemented and studied in various countries requiring the submission of data on existing substances.
In Japan, we handle applications for new chemical substances under Japan’s Act on the Regulation of Manufacture and Evaluation of Chemical Substances and the Industrial Safety and Health Act, as well as quantity notifications under the aforementioned act. In fiscal 2022, the company confirmed the management status of sales and transfer of poisonous and hazardous substances.
With regard to overseas laws and regulations, we continued to respond to formal registration of substances with an annual volume of 1,000 tons or more under the revised K-REACH in South Korea, the country’s Act on Registration and Evaluation, etc. of Chemical Substances. Tosoh also initiated compliance with UK-REACH, the United Kingdom’s law on the registration and evaluation of chemical substances, and REACH notification in Turkey. In fiscal 2022, there were no violations of laws and regulations regarding the registration and notification of chemical substances.
Education on Chemical Substance Regulations
Tosoh Corporation provides education to related divisions and group companies to ensure proper compliance with chemical substance laws and regulations. In January 2009, the European Union (EU) implemented the CLP Regulation, which entails new classification, labeling and packaging regulations for hazardous chemicals. In fiscal 2022, we conducted briefings on this (with about 60 people in attendance), as well as EU safety data sheets (SDSs) online for headquarters, manufacturing complexes, laboratories, and group companies.
Furthermore, we held a briefing (attended by about 120 people) for departments preparing SDSs in Japan regarding compliance with Japan’s revised Act on Confirmation, etc. of Release Amounts of Specific Chemical Substances in the Environment and Promotion of Improvements to the Management Thereof (Law concerning Pollutant Release and Transfer Register (PRTR)).
Enhanced Chemical Substance Management
In order to reach the goal of “aiming to achieve, by 2020, that chemicals are used and produced in ways that lead to the minimization of significant adverse effects on human health and the environment” adopted at the 2002 World Summit on Sustainable Development (WSSD), countries around the world are enacting or amending laws and regulations. The purpose of this is to require chemical manufacturers to manage chemical substances more thoroughly across their supply chains.
In accordance with common global targets set for 2021 and beyond, Tosoh continues to collect information on the enactment and revision of laws and regulations, take appropriate measures, and provide customers with information on chemical substances contained in products. Furthermore, we strive to strengthen chemical substance management and minimize risks throughout the supply chain.
Chemical Substance Management System
Tosoh introduced a chemical substance management system to centrally manage chemicals handled within the company and ensure compliance with international laws and regulations. This system involves the creation of a database of the chemical substances contained in each Tosoh product. The database enables us to search for applicable laws and regulations for the product as well as the chemical substances contained within.
In addition, to enable a rapid response to customers who wish to know the chemical content of those products, Tosoh has certificate-issuing authority for some of its products and can issue and provide response letters or certificates from the department in charge of the particular product. In fiscal 2022, additional products were added, increasing the number of target products from approximately 500 products to around 1,000.
We will continue to improve the system and add new features.
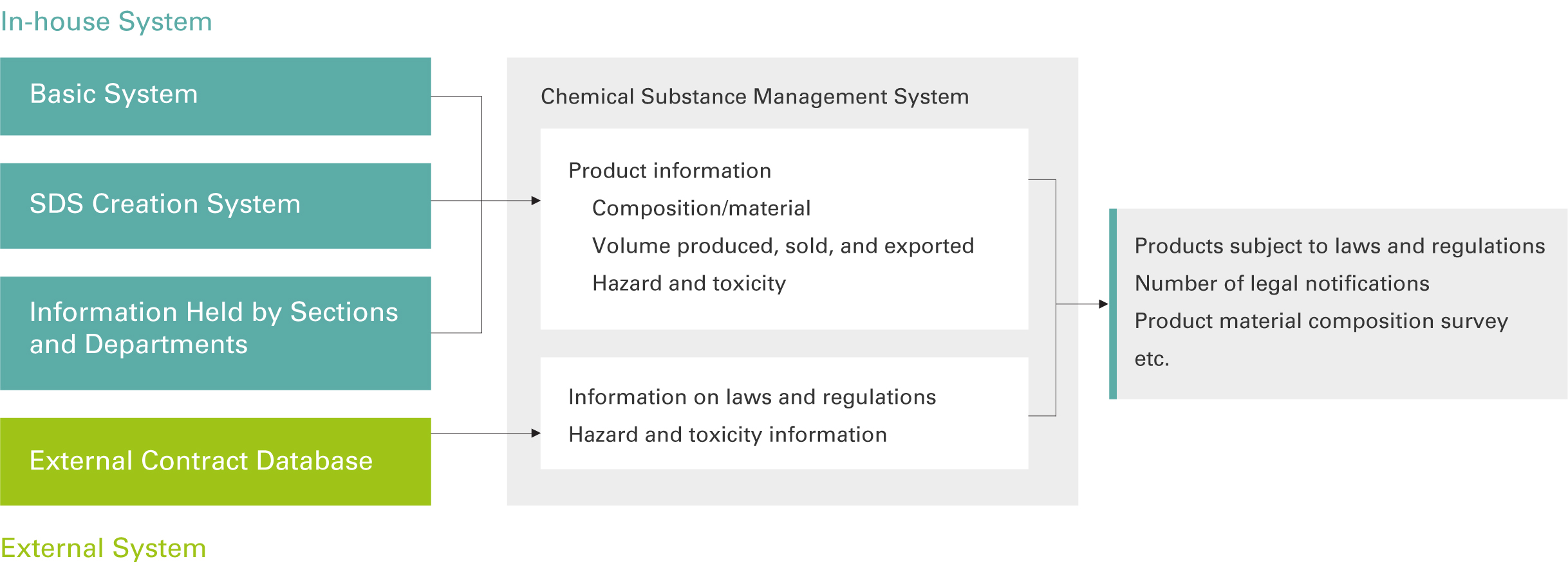
Chemical Substance Risk Management
With the aim of achieving the WSSD’s 2020 targets, the Japan Chemical Industry Association (JCIA) promotes voluntary initiatives by chemical makers in Japan to strengthen chemical management. The initiatives include the Global Product Safety (GPS) strategy and the Japan Initiative of Product Stewardship (JIPS).
Tosoh has been a member of JCIA's GPS/JIPS working group since its establishment, and implements appropriate risk management throughout the entire product lifecycle. The JCIA's focus is to publish safety summaries—summarized results of risk assessments of chemical products manufactured and sold by a company—for 31 major products. When safety data sheets (SDS) describing the chemical content of products and related hazards are revised, the summaries are reviewed and updated accordingly.
In addition, Tosoh appropriately conducted risk assessments for chemical substances handled in the company as required in fiscal 2022 in accordance with Japan’s Industrial Safety and Health Act.
Product Safety Audits
In accordance with the intent of Japan’s Product Liability Act, Tosoh Group employees in Japan work together to ensure product safety and provide appropriate product information.
Our Product Safety Review Committee verifies the safety of raw materials and products and deliberates on laws and regulations at each stage from R&D to sales. In fiscal 2022, the committee met 93 times.
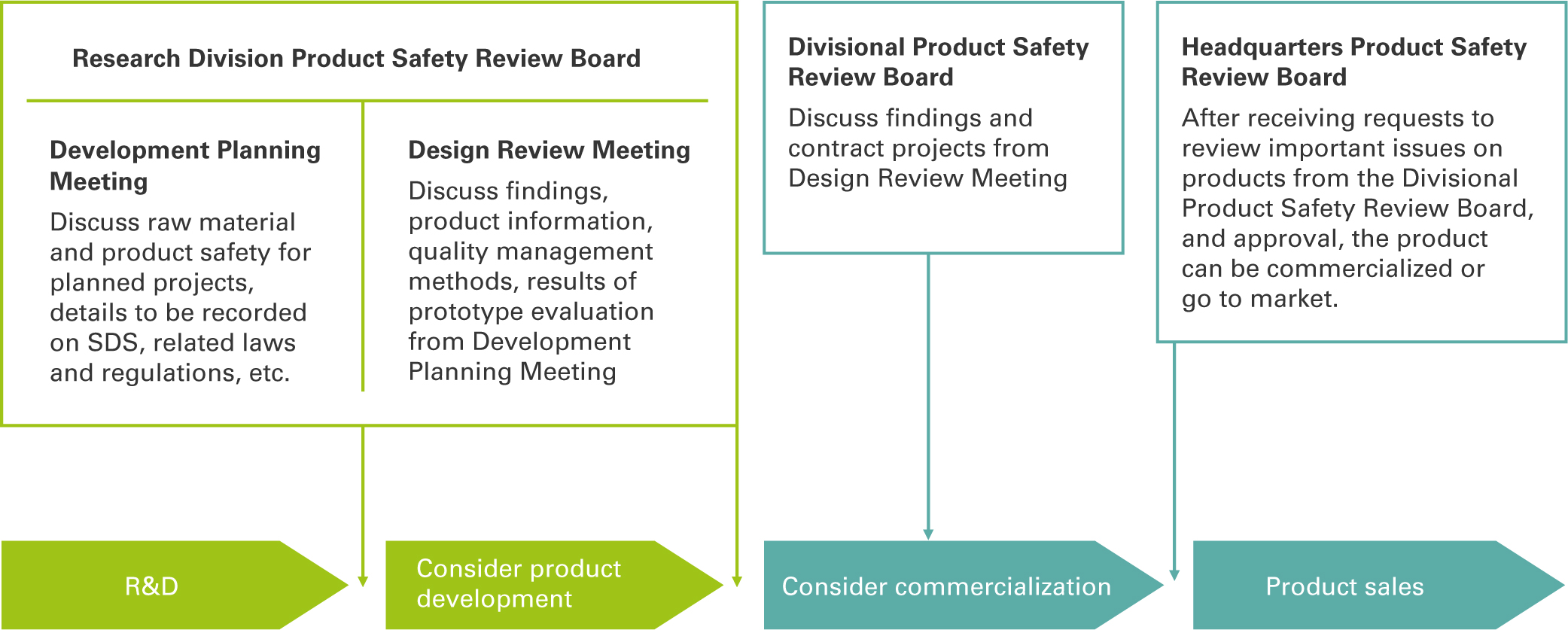
Disclosure of Chemical Substance Safety Information
Safety Information Disclosure
The Tosoh Group prepares and provides SDS for all of its products, and has made some of these available on its website to help ensure the safe handling of the products. Following the Japanese Industrial Standard revisions enacted by the government in May 2019, we completed the revision of all SDSs by May 2022, the deadline for compliance.
As for the labeling of products under Japan’s Industrial Safety and Health Act, in addition to the products that already contain substances subject to labeling, we are gradually including products that contain hazardous substances.*
*Japan's Industrial Safety and Health Act stipulates that containers and packaging for hazardous substances specified by Cabinet Order must be labeled with the name, ingredients, effects on the human body, and precautions for storage and handling.
Dissemination of Information on Chemical Substances Contained in Products
Tosoh is a member company of the Joint Article Management Promotion-consortium (JAMP). Since 2018, we have been promoting the acquisition, management, and communication of information on chemical substances in products in our supply chain using the chemSHERPA system promoted by JAMP. We will continue to provide information in a proactive, prompt, and reliable manner to meet our customers' needs.